Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -isosaccharinic acid
-isosaccharinic acidKobayashi, Taishi*; Sasaki, Takayuki*; Kitamura, Akira
Journal of Chemical Thermodynamics, 138, p.151 - 158, 2019/11
Times Cited Count:3 Percentile:14.74(Thermodynamics)The effect of  -isosaccharinic acid (ISA) on the solubility and redox of tetravalent and hexavalent uranium (U(IV), U(VI)) was investigated in the hydrogen ion concentration (pH
-isosaccharinic acid (ISA) on the solubility and redox of tetravalent and hexavalent uranium (U(IV), U(VI)) was investigated in the hydrogen ion concentration (pH ) range of 6
) range of 6 13 and at total ISA concentration ([ISA]
13 and at total ISA concentration ([ISA]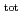 ) = 10
) = 10
 10
10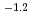 mol/dm
mol/dm . The dependence of U(IV) solubility on pH
. The dependence of U(IV) solubility on pH and [ISA]
and [ISA]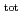 suggested the existence of U(OH)
suggested the existence of U(OH) (ISA)
(ISA)
 as a dominant species within the investigated pH
as a dominant species within the investigated pH range of 6
range of 6 12. For the U(VI)-ISA system, UO
12. For the U(VI)-ISA system, UO (OH)
(OH) (ISA)
(ISA)
 was suggested as a dominant species at pH
was suggested as a dominant species at pH 7
7 13. The formation constants of the U(IV)-ISA and U(VI)-ISA complexes were determined by least-squares fitting of the solubility data. The solubility of U(IV) and U(VI) in the presence of ISA and its effect on the redox behavior were thermodynamically interpreted based on the obtained constants.
13. The formation constants of the U(IV)-ISA and U(VI)-ISA complexes were determined by least-squares fitting of the solubility data. The solubility of U(IV) and U(VI) in the presence of ISA and its effect on the redox behavior were thermodynamically interpreted based on the obtained constants.
;
Journal of Nuclear Science and Technology, 15(9), p.685 - 689, 1978/09
no abstracts in English
;
Radiochem.Radioanal.Lett., 29(6), p.331 - 340, 1977/06
no abstracts in English